Outrageous Tips About How To Tell If A Compound Is Polar

You can also make a rough estimate of how polar it will be.
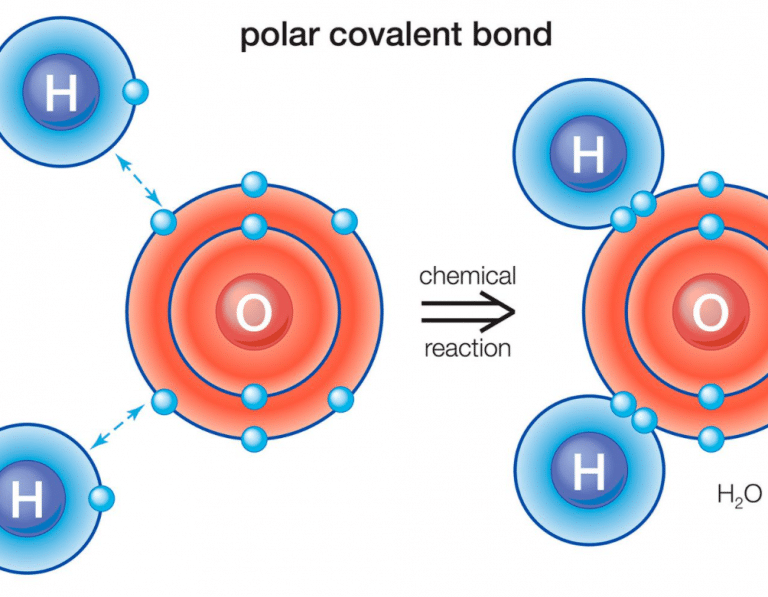
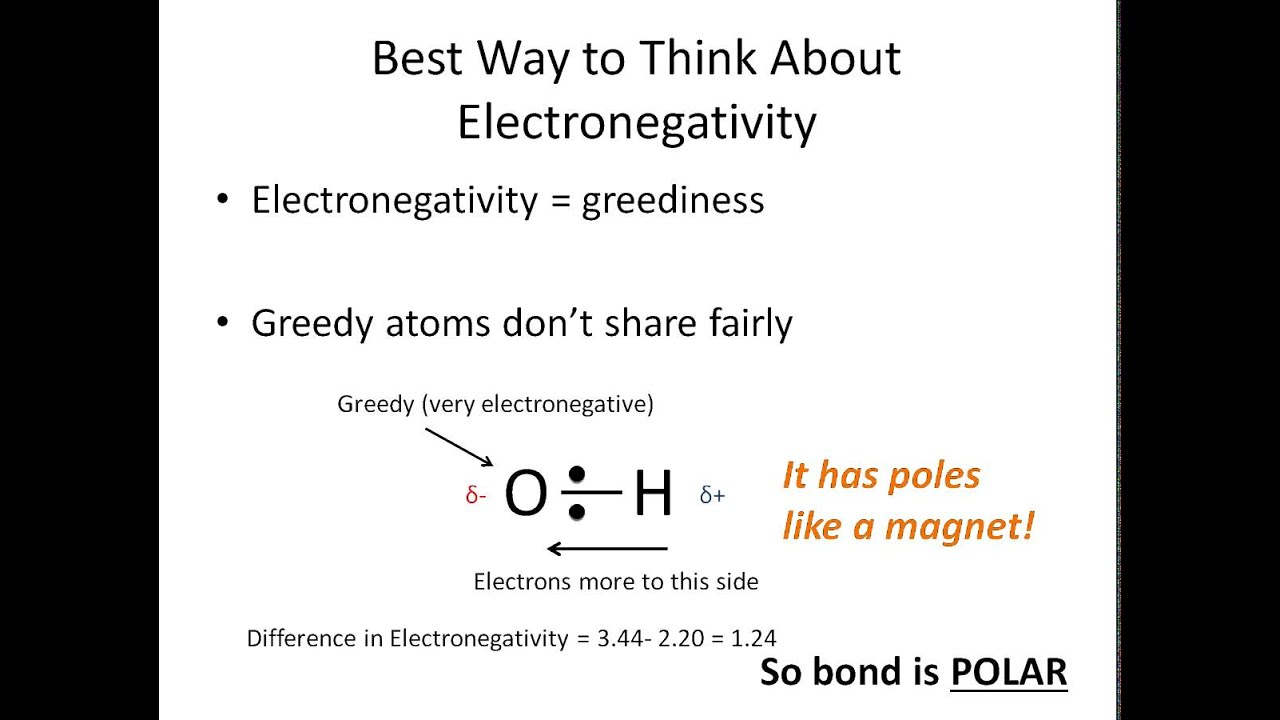
How to tell if a compound is polar. A diatomic molecule that consists of a polar covalent bond, such as \(\ce{hf}\), is a polar molecule. This video provides a fast way for you to determine if a molecule is polar or nonpolar. If the difference of the electronegativity between the two elements is greater than 1.7 then the bond is ionic.
2.5m views 8 years ago. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and. Assess the polarity of a molecule based on its bonding and structure.
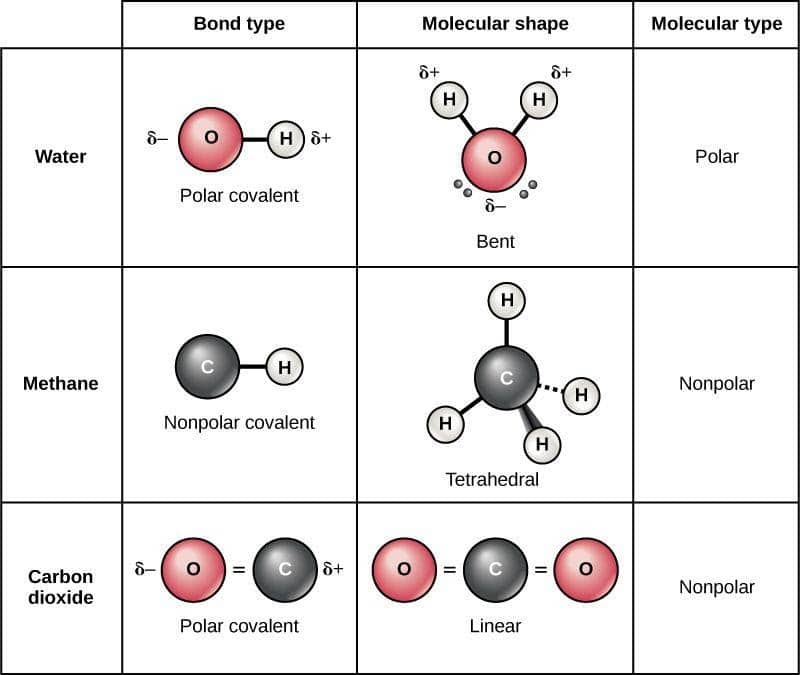
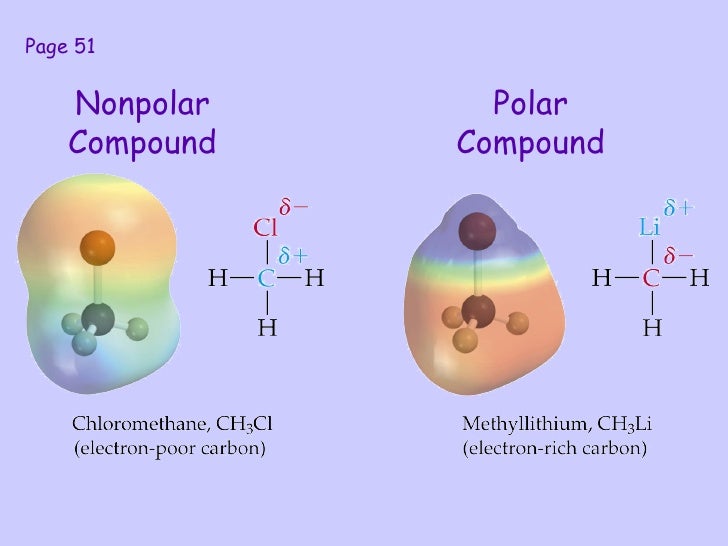
The difference with a polar covalent. In short, the molecule itself is polar. A compound is polar if one part of it has a partial positive charge and the other part has a partial negative charge.
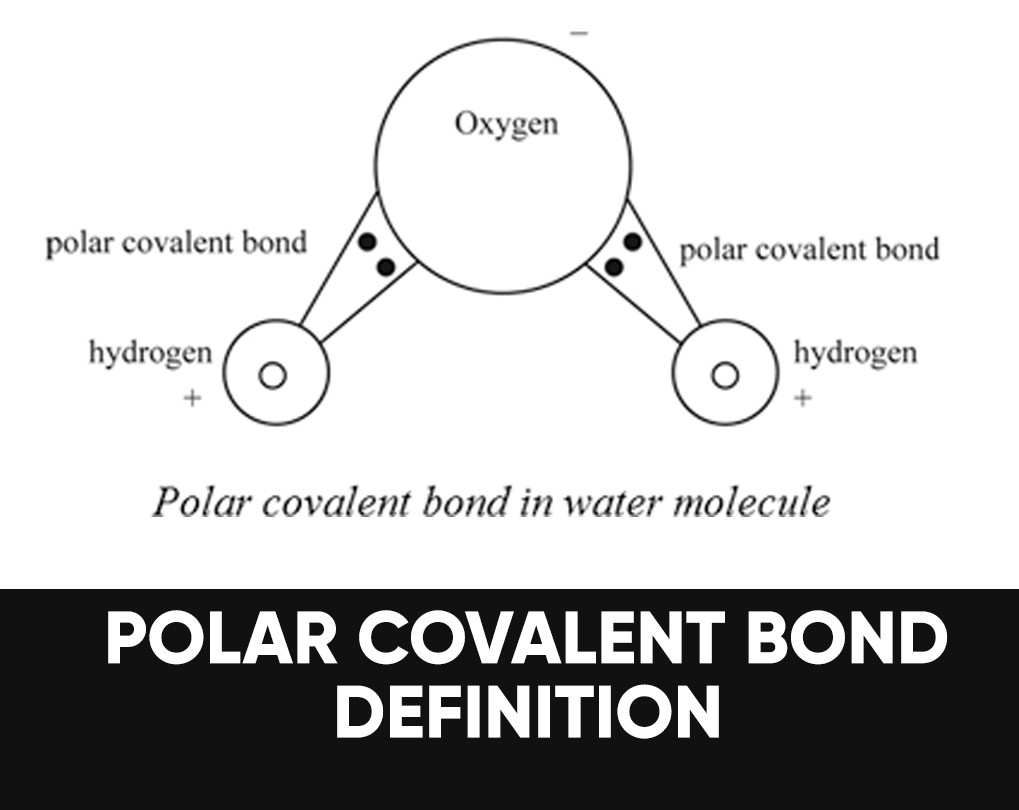
Polar bonds do not share electrons equally, meaning the. The polarity of water has an enormous impact on its physical and chemical properties. (for example, the boiling point of water.
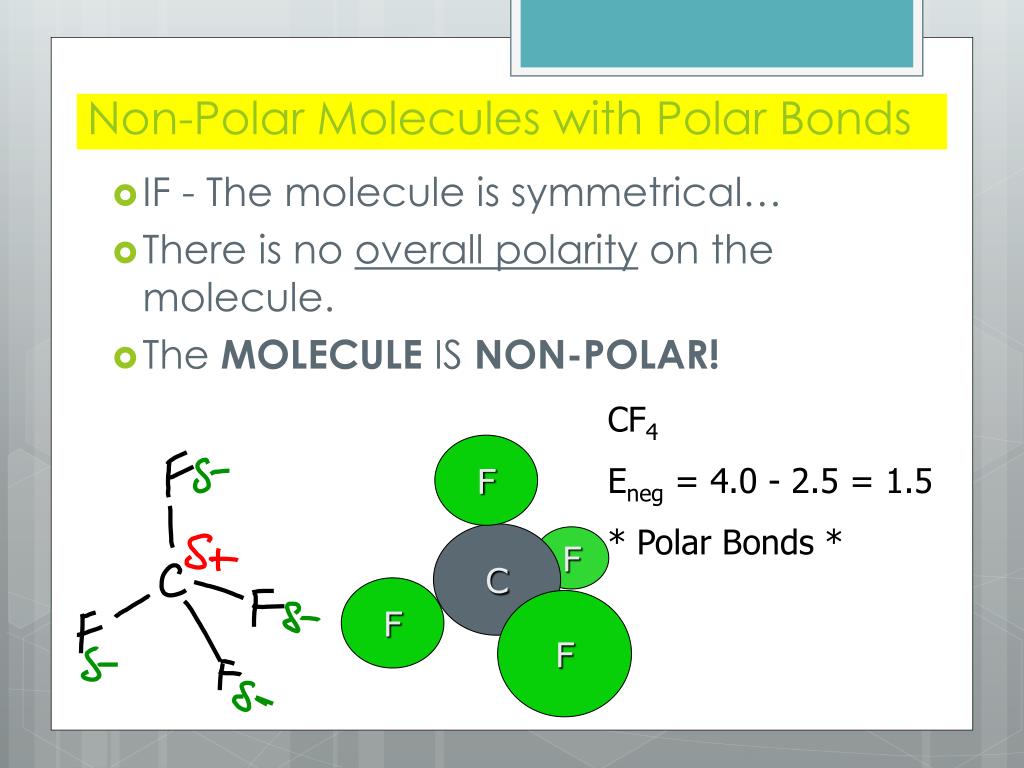
The bond type, electronegativity, and molecular. The organic chemistry tutor. Using this rule together with vsepr theory, you can predict whether a molecule is polar or not.
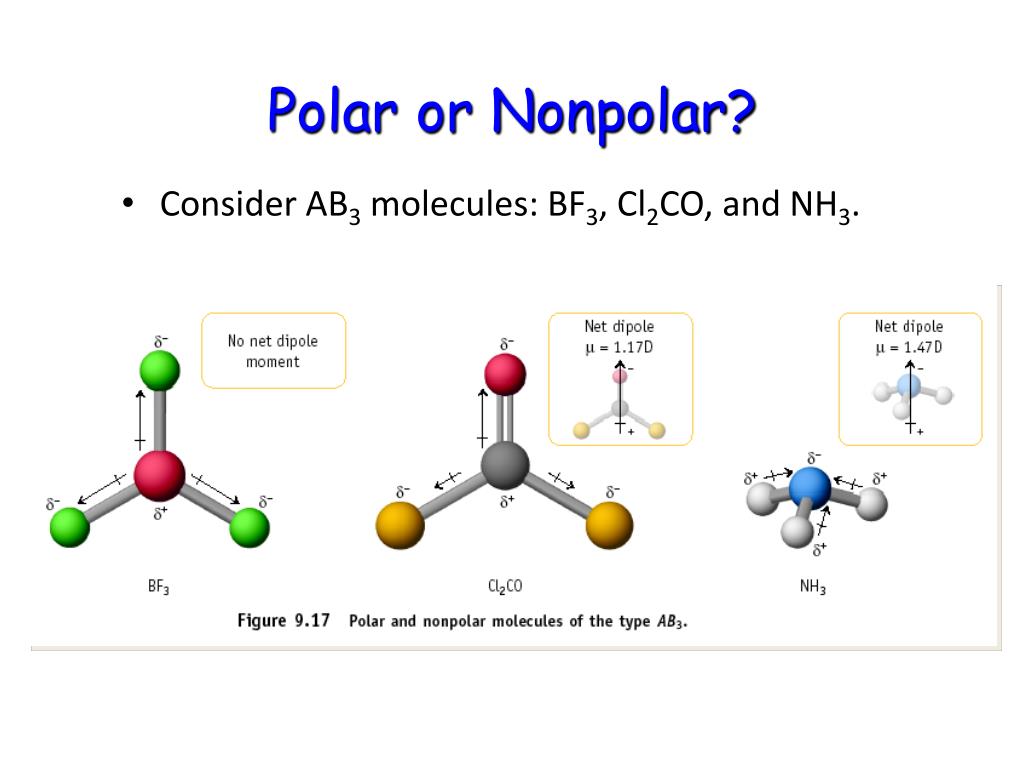
A trigonal planar molecule (bf3) ( bf 3) may be nonpolar if all three peripheral atoms are the same, but a trigonal pyramidal molecule (nh3) ( nh 3) is polar. Polar compounds are chemical compounds that are held together. Like bonds, molecules can also be polar.
A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. If a charge is evenly distributed around a central. This video discusses how to tell if a molecule / compound is polar or nonpolar.
If one end of the molecule has a positive charge while the other end has a negative charge, the molecule is polar. Learn how to identify polar and nonpolar molecules based on their covalent and ionic bonds, electronegativity, and lewis structure. As mentioned in section 4.7, because the electrons in the bond are nearer to the f atom, this side of the molecule takes on a partial negative.